HICKS Lab
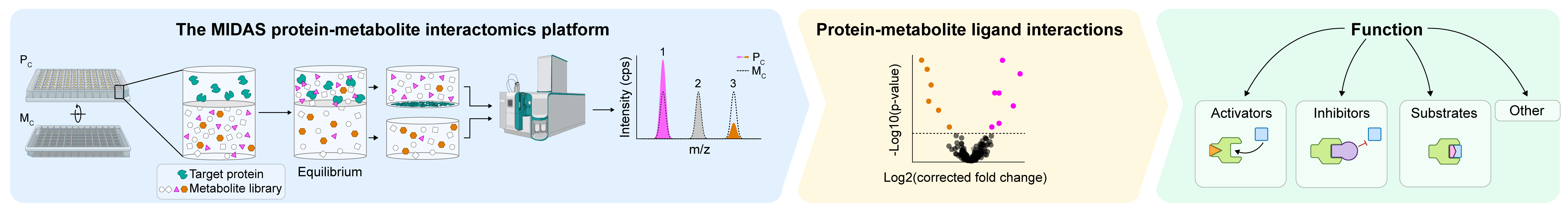
The MIDAS Platform
Metabolites are conventionally viewed as the elementary constituents of life, functioning as cellular building blocks, energy currency, and waste products. Beyond anabolic and catabolic metabolism, it is increasingly evident that metabolites serve as signaling molecules, directly regulating protein function to provide a rapid and adaptive mechanism for metabolism to coordinate diverse cellular processes that are energetically or biosynthetically demanding. Furthermore, in healthy tissues, metabolism is internally balanced through evolved protein-metabolite interactions, more specifically, metabolite-mediated regulation of enzyme activity. Aberrant dysregulation of these regulatory protein-metabolite interactions likely contributes to the mechanisms driving many metabolically-dependent diseases including cancer, diabetes, heart disease, and inborn errors of metabolism. A deeper understanding of endogenous metabolite interactions with disease-relevant proteins will also benefit allosteric drug discovery. The true scale and function of the protein-metabolite interactome is unknown and represents a major void in our understanding of cellular biology and metabolism.
To begin to reveal the protein-metabolite interactome, we developed the MIDAS platform to systematically identify interactions between proteins and metabolites (DOI: 10.1126/science.abm3452). The MIDAS platform is uniquely situated to identify low millimolar and stronger binding affinities between soluble proteins and metabolite ligands from a multiplexed metabolite library representative of the human metabolome. As such, the MIDAS platform is a powerful tool to discover and investigate the form and function of the interactions spanning the proteome and metabolome.
University of Utah 2024